Semaglutide
Wegovy semaglutide is a once-weekly injectable prescription medication that can assist patients with long-term weight management. Semaglutide is an effective treatment for type 2 diabetes.

Semaglutide What Is Semaglutide About Its Science Chemistry And Structure
Semaglutide is in a class of medications called incretin mimetics.

. Semaglutide is a glucagon-like peptide-1 GLP-1 analogue in phase 3 development for type 2 diabetes. GLP-1 is produced in the body after eating a meal. Along with diet and exercise to improve blood sugar in adults with type 2 diabetes.
It works by causing insulinrelease in response to high bloodsugar such as after a meal and decreasing the. Semaglutide rybelsus for the treatment of adults with insufficiently controlled type 2 diabetes to improve glycaemic control as an adjunct to diet and exercise. The Food and Drug Administration FDA approved semaglutide brand name Wegovy for use in obesity.
Semaglutide is one of the medicines that are prescribed for people with type 2 diabetes. Semaglutide C187H291N45O59 CID 56843331 - structure chemical names physical and chemical properties classification patents literature biological activities. Wegovy semaglutide injection 24 mg is an injectable prescription medicine used for adults with obesity BMI 30 or overweight excess weight BMI 27 who also have weight-related.
Ozempic semaglutide injection 05 mg 1 mg or 2 mg is an injectable prescription medicine used. Semaglutide is the generic name for Ozempic Rybelsus and Wegovy. However 20-30 of patients given semaglutide 10 mg do not reach glycaemic treatment goals.
It works by helping the pancreas to release the right amount of insulin when blood sugar levels are high. Drugs belonging to the GLP-1 drug. Semaglutide is similar to a natural hormonein your body incretin.
Semaglutide is a prescription medication that is used to treat obesity. We assessed the efficacy safety and tolerability of semaglutide monotherapy. Semaglutide injection Wegovy is used along with an individualized low-calorie low-fat diet and exercise program to help with weight loss in overweight adults who may also have high blood.
Novo Nordisk manufactures all three brand-name medications. Semaglutide is a medicine which is similar to a natural hormone called glucagon-like peptide-1 GLP-1. Find information about common infrequent and rare side effects of semaglutide subcutaneous.
This treatment works to facilitate weight loss in. Ozempic and Rybelsus are FDA. Controlling high blood sugar helps prevent kidney damage blindness.
Semaglutide very closely resembles a natural hormone called glucagon-like peptide-1. Semaglutide is used with a proper diet and exercise program to control high blood sugar in people with type 2 diabetes.
Wegovy Semaglutide For Chronic Weight Management

Semaglutide What Does It Do Antidotehealth Ai

Once Weekly Semaglutide In Adults With Overweight Or Obesity Nejm
Novo Nordisk S Semaglutide Hits Mark In Type 2 Diabetes Trial Biospace
Buy Semaglutide 3mg Ozempic Genx Peptides

New Weight Loss Medication Trial Semaglutide Medication Management Llc

Tirzepatide Versus Semaglutide For Type 2 Diabetes Nejm

Dosing Prescribing Ozempic Semaglutide Injection 0 5 Mg 1 Mg Or 2 Mg

1500 A Month Breakthrough Obesity Injection Wegovy Semaglutide Approved By The Fda But High Costs And Limited Insurance Coverage Likely To Limit Adoption Genetic Literacy Project

Ozempic Semaglutide For The Treatment Of Type 2 Diabetes Clinical Trials Arena

Semaglutide Peptide Therapy Las Vegas American Male Wellness Clinic
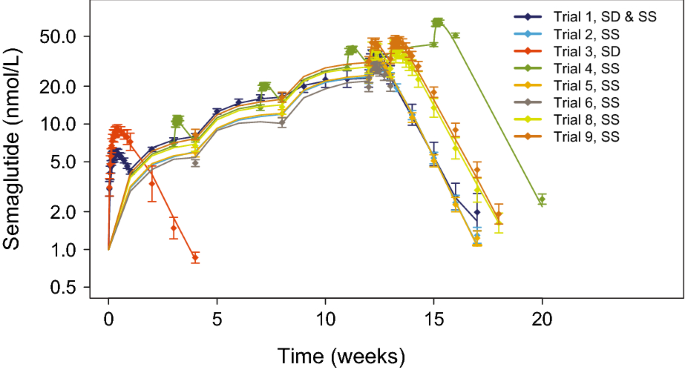
Population Pharmacokinetics Of Semaglutide For Type 2 Diabetes Springerlink

Semaglutide Effective Medical Weight Loss Peak Medical

Semaglutide A Weight Loss Breakthrough Scottsdale Weight Loss Center

Diabetes Drug Semaglutide Delivers Stunning Weight Loss Results

Weight Loss Drug Wegovy Exceeded Expectations And Supply Medpage Today
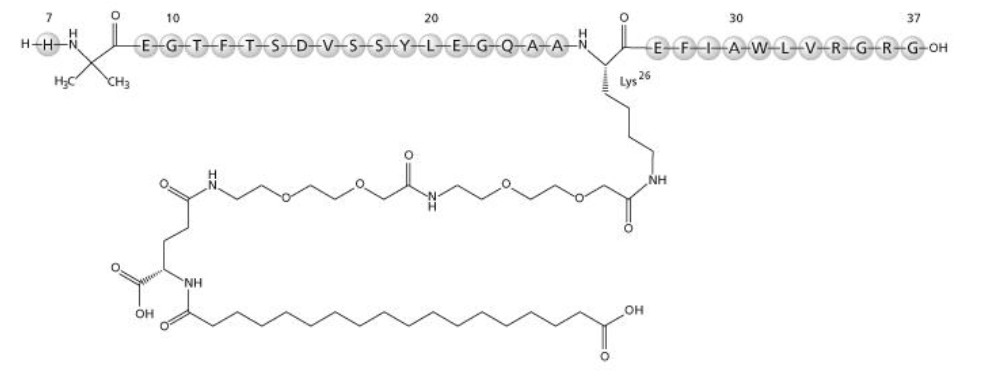
Semaglutide Api Manufacturer And Supplier Cas 910463 68 2 Dr Reddy S

Oral Semaglutide Versus Subcutaneous Liraglutide And Placebo In Type 2 Diabetes Pioneer 4 A Randomised Double Blind Phase 3a Trial The Lancet
